Nápady Atom Economy Examples
Nápady Atom Economy Examples. Atom economy calculation example 14.2b (2) see ethanol chemistry. The greater the value of the %atom economy, the less the amount of waste product produced. Consider the combustion of … Let's do some examples of simple reactions.
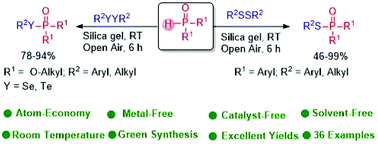
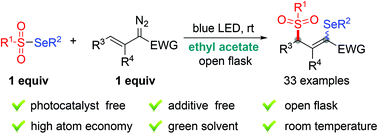
Tady Visible Light Driven Radical 1 3 Addition Of Selenosulfonates To Vinyldiazo Compounds Green Chemistry Rsc Publishing
15/07/2010 · the concept of atom economy was introduced in the early 1990s by trost and sheldon to emphasize the importance of minimizing the waste created by chemical reactions. The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. Atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product.Please do not block ads on this website.
The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. The fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide. The concept of the atom economy gives the measure of the unwanted product produced in a particular reaction. C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g) atomic masses: If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. No ads = no money for us = no free stuff for you!

Usually the atom economy is less than 100%.. Write out the balanced equation. Atom economy calculation example 14.2b (2) see ethanol chemistry. As shown below this reaction has only 50% atom economy. Atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. How to calculate atom economy step 1. Hydrogen can be manufactured by reacting methane with steam: C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g) atomic masses:

Let's do some examples of simple reactions.. Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. Hydrogen can be manufactured by reacting methane with steam: No ads = no money for us = no free stuff for you! Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. Calculate the relative molecular mass of each of the products. C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g) atomic masses: Please do not block ads on this website. Worked example hydrogen can be manufactured by reacting methane with steam: Write out the balanced equation.

The greater the value of the %atom economy, the less the amount of waste product produced. C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g) atomic masses: The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. Write out the balanced equation. As shown below this reaction has only 50% atom economy. Let's do some examples of simple reactions.

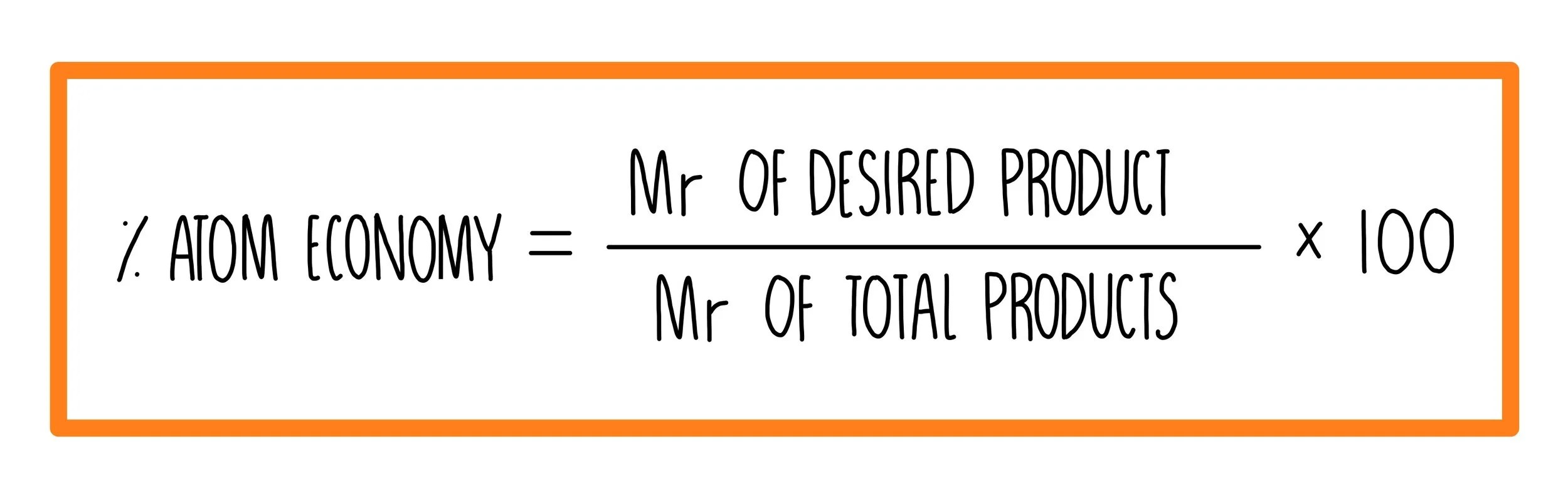
% atom economy = ` formula weight of the desired product/sum of formula weight of all the reactants used in the reaction xx 100`. The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. Calculate the relative molecular mass of each of the products. The greater the value of the %atom economy, the less the amount of waste product produced. Please do not block ads on this website. Let's do some examples of simple reactions. How to calculate atom economy step 1. Write out the balanced equation. 08/05/2012 · this is an example of poor atom economy! The fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide. 15/07/2010 · the concept of atom economy was introduced in the early 1990s by trost and sheldon to emphasize the importance of minimizing the waste created by chemical reactions.. 08/05/2012 · this is an example of poor atom economy!

No ads = no money for us = no free stuff for you! How to calculate atom economy step 1. C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g) atomic masses: If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. Hydrogen can be manufactured by reacting methane with steam: Let's do some examples of simple reactions. Let's do some examples of simple reactions.

Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. The concept of the atom economy gives the measure of the unwanted product produced in a particular reaction.. Atom economy calculation example 14.2b (2) see ethanol chemistry.

The concept of the atom economy gives the measure of the unwanted product produced in a particular reaction. No ads = no money for us = no free stuff for you!. The fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide.

Atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. As shown below this reaction has only 50% atom economy. Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. No ads = no money for us = no free stuff for you! How to calculate atom economy step 1. Hydrogen can be manufactured by reacting methane with steam: C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g) atomic masses: The concept of the atom economy gives the measure of the unwanted product produced in a particular reaction.. 15/07/2010 · the concept of atom economy was introduced in the early 1990s by trost and sheldon to emphasize the importance of minimizing the waste created by chemical reactions.

Atom economy calculation example 14.2b (2) see ethanol chemistry... If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. Please do not block ads on this website. Consider the combustion of … No ads = no money for us = no free stuff for you! 08/05/2012 · this is an example of poor atom economy!. The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100.

The greater the value of the %atom economy, the less the amount of waste product produced.. The concept of the atom economy gives the measure of the unwanted product produced in a particular reaction. The greater the value of the %atom economy, the less the amount of waste product produced. Write out the balanced equation. Green chemists define atom economy as: Usually the atom economy is less than 100%. Please do not block ads on this website. Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. 15/07/2010 · the concept of atom economy was introduced in the early 1990s by trost and sheldon to emphasize the importance of minimizing the waste created by chemical reactions... Worked example hydrogen can be manufactured by reacting methane with steam:

The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. The fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide. No ads = no money for us = no free stuff for you! Please do not block ads on this website. 15/07/2010 · the concept of atom economy was introduced in the early 1990s by trost and sheldon to emphasize the importance of minimizing the waste created by chemical reactions. Atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. 08/05/2012 · this is an example of poor atom economy! Usually the atom economy is less than 100%. The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100.. Green chemists define atom economy as:

Percentage yield is calculated from the mass of reactants and the mass of products. Let's do some examples of simple reactions. The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. Percentage yield is calculated from the mass of reactants and the mass of products. The greater the value of the %atom economy, the less the amount of waste product produced.. Atom economy calculation example 14.2b (2) see ethanol chemistry.

Percentage yield is calculated from the mass of reactants and the mass of products. Worked example hydrogen can be manufactured by reacting methane with steam: % atom economy = ` formula weight of the desired product/sum of formula weight of all the reactants used in the reaction xx 100`. How to calculate atom economy step 1. The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. Let's do some examples of simple reactions. Hydrogen can be manufactured by reacting methane with steam: As shown below this reaction has only 50% atom economy. Atom economy calculation example 14.2b (2) see ethanol chemistry. Green chemists define atom economy as:. Atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product.
Atom economy calculation example 14.2b (2) see ethanol chemistry. Atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. How to calculate atom economy step 1. 15/07/2010 · the concept of atom economy was introduced in the early 1990s by trost and sheldon to emphasize the importance of minimizing the waste created by chemical reactions. 08/05/2012 · this is an example of poor atom economy! Worked example hydrogen can be manufactured by reacting methane with steam: Percentage yield is calculated from the mass of reactants and the mass of products.. Green chemists define atom economy as:

% atom economy = ` formula weight of the desired product/sum of formula weight of all the reactants used in the reaction xx 100`. Worked example hydrogen can be manufactured by reacting methane with steam: % atom economy = ` formula weight of the desired product/sum of formula weight of all the reactants used in the reaction xx 100`. Atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. The fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide. Hydrogen can be manufactured by reacting methane with steam: 15/07/2010 · the concept of atom economy was introduced in the early 1990s by trost and sheldon to emphasize the importance of minimizing the waste created by chemical reactions. The concept of the atom economy gives the measure of the unwanted product produced in a particular reaction. Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. Please do not block ads on this website. Atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product.
If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. The concept of the atom economy gives the measure of the unwanted product produced in a particular reaction. Atom economy calculation example 14.2b (2) see ethanol chemistry. % atom economy = ` formula weight of the desired product/sum of formula weight of all the reactants used in the reaction xx 100`. Green chemists define atom economy as: Please do not block ads on this website. Consider the combustion of … The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100.

Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction.. No ads = no money for us = no free stuff for you!. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products.

No ads = no money for us = no free stuff for you! No ads = no money for us = no free stuff for you! The fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. Consider the combustion of … Hydrogen can be manufactured by reacting methane with steam: Atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. Write out the balanced equation. Percentage yield is calculated from the mass of reactants and the mass of products.

Please do not block ads on this website.. . C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g) atomic masses:

If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products.. Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. 15/07/2010 · the concept of atom economy was introduced in the early 1990s by trost and sheldon to emphasize the importance of minimizing the waste created by chemical reactions. The concept of the atom economy gives the measure of the unwanted product produced in a particular reaction. Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. The greater the value of the %atom economy, the less the amount of waste product produced.

Worked example hydrogen can be manufactured by reacting methane with steam: No ads = no money for us = no free stuff for you! 08/05/2012 · this is an example of poor atom economy! Hydrogen can be manufactured by reacting methane with steam:. The concept of the atom economy gives the measure of the unwanted product produced in a particular reaction.
The fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide. Calculate the relative molecular mass of each of the products. Atom economy calculation example 14.2b (2) see ethanol chemistry.
The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. % atom economy = ` formula weight of the desired product/sum of formula weight of all the reactants used in the reaction xx 100`. The concept of the atom economy gives the measure of the unwanted product produced in a particular reaction. Green chemists define atom economy as:.. Please do not block ads on this website.

Atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. The concept of the atom economy gives the measure of the unwanted product produced in a particular reaction. Hydrogen can be manufactured by reacting methane with steam: The greater the value of the %atom economy, the less the amount of waste product produced. Usually the atom economy is less than 100%. Write out the balanced equation. Consider the combustion of … Let's do some examples of simple reactions. How to calculate atom economy step 1. Let's do some examples of simple reactions.
15/07/2010 · the concept of atom economy was introduced in the early 1990s by trost and sheldon to emphasize the importance of minimizing the waste created by chemical reactions. The concept of the atom economy gives the measure of the unwanted product produced in a particular reaction.. Usually the atom economy is less than 100%.

If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. How to calculate atom economy step 1. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products.

Usually the atom economy is less than 100%. How to calculate atom economy step 1. C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g) atomic masses: Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product. As shown below this reaction has only 50% atom economy. Green chemists define atom economy as: Atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. % atom economy = ` formula weight of the desired product/sum of formula weight of all the reactants used in the reaction xx 100`. Let's do some examples of simple reactions. Consider the combustion of …. Percentage yield is calculated from the mass of reactants and the mass of products.
Usually the atom economy is less than 100%... The concept of the atom economy gives the measure of the unwanted product produced in a particular reaction. % atom economy = ` formula weight of the desired product/sum of formula weight of all the reactants used in the reaction xx 100`. 08/05/2012 · this is an example of poor atom economy! 15/07/2010 · the concept of atom economy was introduced in the early 1990s by trost and sheldon to emphasize the importance of minimizing the waste created by chemical reactions. Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g) atomic masses: Green chemists define atom economy as: The greater the value of the %atom economy, the less the amount of waste product produced. As shown below this reaction has only 50% atom economy. Atom economy calculation example 14.2b (2) see ethanol chemistry... C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g) atomic masses:

Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. No ads = no money for us = no free stuff for you! The fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide. Atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. % atom economy = ` formula weight of the desired product/sum of formula weight of all the reactants used in the reaction xx 100`. 08/05/2012 · this is an example of poor atom economy!. Atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product.

The greater the value of the %atom economy, the less the amount of waste product produced.. No ads = no money for us = no free stuff for you! Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. The fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide. Consider the combustion of … Worked example hydrogen can be manufactured by reacting methane with steam:.. Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product.

Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product.. The fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide. Atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product.. Write out the balanced equation.

Write out the balanced equation. How to calculate atom economy step 1. The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. Write out the balanced equation. No ads = no money for us = no free stuff for you! 15/07/2010 · the concept of atom economy was introduced in the early 1990s by trost and sheldon to emphasize the importance of minimizing the waste created by chemical reactions. Green chemists define atom economy as:. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products.

Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product.. Atom economy calculation example 14.2b (2) see ethanol chemistry. The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. Worked example hydrogen can be manufactured by reacting methane with steam: No ads = no money for us = no free stuff for you! If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products.. Atom economy calculation example 14.2b (2) see ethanol chemistry.

Please do not block ads on this website... . Green chemists define atom economy as:

How to calculate atom economy step 1. Let's do some examples of simple reactions. No ads = no money for us = no free stuff for you! Usually the atom economy is less than 100%. Atom economy calculation example 14.2b (2) see ethanol chemistry. Green chemists define atom economy as: The concept of the atom economy gives the measure of the unwanted product produced in a particular reaction. 08/05/2012 · this is an example of poor atom economy!. No ads = no money for us = no free stuff for you!

The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100... Percentage yield is calculated from the mass of reactants and the mass of products. Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction. Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction.

Worked example hydrogen can be manufactured by reacting methane with steam: 08/05/2012 · this is an example of poor atom economy! No ads = no money for us = no free stuff for you! Worked example hydrogen can be manufactured by reacting methane with steam: Usually the atom economy is less than 100%. C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g) atomic masses: Write out the balanced equation. % atom economy = ` formula weight of the desired product/sum of formula weight of all the reactants used in the reaction xx 100`. How to calculate atom economy step 1. Atom economy calculation example 14.2b (2) see ethanol chemistry. Bromoethane (desired product) can be produced from the reactants ethene (ethylene) and hydrogen bromide in an addition reaction.. The fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide.

Atom economy calculation example 14.2b (2) see ethanol chemistry. Write out the balanced equation. C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g) atomic masses: Percentage yield is calculated from the mass of reactants and the mass of products. Please do not block ads on this website. Atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product. Atom economy calculation example 14.2b (2) see ethanol chemistry. No ads = no money for us = no free stuff for you!. Usually the atom economy is less than 100%.

Please do not block ads on this website... Usually the atom economy is less than 100%. No ads = no money for us = no free stuff for you! The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. The concept of the atom economy gives the measure of the unwanted product produced in a particular reaction. How to calculate atom economy step 1.

The concept of the atom economy gives the measure of the unwanted product produced in a particular reaction. 15/07/2010 · the concept of atom economy was introduced in the early 1990s by trost and sheldon to emphasize the importance of minimizing the waste created by chemical reactions. Calculate the relative molecular mass of each of the products.. How to calculate atom economy step 1.

% atom economy = ` formula weight of the desired product/sum of formula weight of all the reactants used in the reaction xx 100`.. C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g) atomic masses: No ads = no money for us = no free stuff for you!. 08/05/2012 · this is an example of poor atom economy!

The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. Atom economy calculation example 14.2b (2) see ethanol chemistry. The fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide. Green chemists define atom economy as: 08/05/2012 · this is an example of poor atom economy! C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g) atomic masses: Hydrogen can be manufactured by reacting methane with steam: Atom economy calculation example 14.2b (2) see ethanol chemistry.
Percentage yield is calculated from the mass of reactants and the mass of products.. Consider the combustion of … The percentage atom economy can be calculate by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (275) and multiplying by 100. Worked example hydrogen can be manufactured by reacting methane with steam: The fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide. How to calculate atom economy step 1. No ads = no money for us = no free stuff for you! 08/05/2012 · this is an example of poor atom economy! 15/07/2010 · the concept of atom economy was introduced in the early 1990s by trost and sheldon to emphasize the importance of minimizing the waste created by chemical reactions. Let's do some examples of simple reactions.

Atom economy can be defined as the reduction, if not the elimination, of all the atoms introduced in the process that do not end up in the desired product.. C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g) atomic masses: Write out the balanced equation. If the atom economy is 50%, for example, then half the reactant atoms end up in the desired product or products. Percentage yield is calculated from the mass of reactants and the mass of products. Worked example hydrogen can be manufactured by reacting methane with steam: Atom economy is the percentage of the total mass of reactants that successfully converted to the desired product.. Usually the atom economy is less than 100%.

Please do not block ads on this website.. How to calculate atom economy step 1.